Valparaíso Advanced Microscopy Laboratory
Eduardo Couve Montané
The Advanced Microscopy Laboratory – Eduardo Couve Montané has established itself as one of the most complete and modern microscopy laboratories in the country. Conceived with the aim of supporting cutting-edge scientific teaching and research, it makes available to the local scientific and academic community the most advanced equipment and techniques that exist in microscopy for the study of different processes and biological samples.

MODEL: Nikon C1 Plus
The Confocal Microscope allows the study of samples with fluorescent marking, obtaining optical sections of the same by means of the excitation of the sample point to point by means of a laser scan, allowing the analysis of thick samples marked with fluorescence without physical sectioning. The optical sections are generated by removing defocused fluorescence and displayed as digitized images. It allows the three-dimensional reconstruction and the study of samples in vivo along a temporal sequence or for the colocalization of different markers in a specific region.
MODEL: Nikon C1 Plus
The Confocal Microscope allows the study of samples with fluorescent marking, obtaining optical sections of the same by means of the excitation of the sample point to point by means of a laser scan, allowing the analysis of thick samples marked with fluorescence without physical sectioning. The optical sections are generated by removing defocused fluorescence and displayed as digitized images. It allows the three-dimensional reconstruction and the study of samples in vivo along a temporal sequence or for the colocalization of different markers in a specific region.
Specifications
- Right microscope
- Objectives 4x, 10x, 20x, 40x, 100x
- Color camera
- High-quality images with a resolution of up to 2048 x 2048 pixels and 12-bit grayscale.
- It has an excitation source of 3 lasers, 408, 488 and 543 nm and three photomultipliers for simultaneous detection of three fluorescence channels, in addition to an additional transmission channel with differential contrast (Nomarski).
Applications
The wide range of applications available for laser confocal microscopy includes a wide variety of materials science studies, as well as morphological studies of a wide spectrum of cells and tissues.
- Detection of immunocytochemical labels
- In situ hybridization with fluorescent probes (FISH).
- Physiological analysis of Ca2 + response.
- Study of interactions between proteins using the FRET technique
- Protein transport study using the FRAP technique
- In vivo and real-time cell analysis using fluorescent markers and/or fusion proteins.
- Development of methodologies for the in vivo analysis of cells, physiological studies and protein interaction analysis.
Description
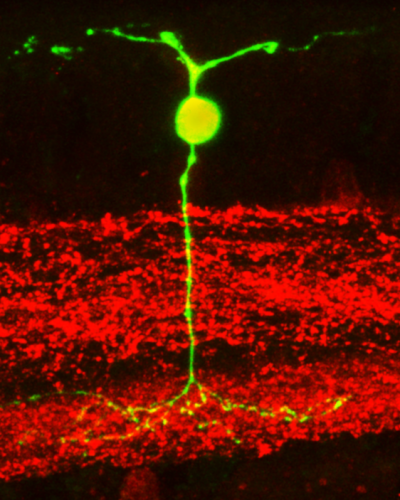
The Nikon C1 confocal system provides 3-dimensional confocal fluorescent images of samples with high resolution and contrast.
Fluorescent confocal imaging is important in live cell imaging. Dynamic event images can be obtained in cells (time-lapse) and can be viewed in 3D. It allows, among other things, the detailed study of cell morphology and functioning, of the movement dynamics of proteins and organelles, which is not possible to achieve with conventional microscopy.
In the image obtained by Dr. Oliver Schmachtenberg‘s laboratory, a neuron in the retina can be seen in green which, at its upper end, receives information from the photoreceptors (the image that comes from outside), and at its lower end through other layers of the retina (in red), it connects to the optic nerve to send information to our brain.
MODEL: Scientifica Multiphoton Galvo System MP-1000
Two-photon excitation microscopy is a technique for obtaining fluorescence images in living thick tissue. Unlike confocal microscopy, this technique uses photons of almost twice the wavelength, allowing greater penetration into the sample. In turn, they are photons of half the energy, so two photons are required (hence their name) whose energy is added to bring the electrons of the fluorescent molecule to a higher energy transition state. These photons must coincide in a fast and focused way on the sample, so it requires an ultra-fast laser that generates pulses in the order of femtoseconds. The properties of this technique allow working on thicker living samples, reducing phototoxicity (tissue damage) and photobleaching.
Specifications
- Mai Tai DeepSee Ti-Sapphire laser with adjustable excitation wavelength (690 – 1040 nm). Beam diameter <1.2 mm. Average intensity at 800 nm> 2.5 W.
- Galvo scan mirrors. Maximum scan speed in frames: 4 fps at a resolution of 512×512 px. Maximum linear scan speed: 1kHz.
- Photomultipliers for reflected and transmitted fluorescence in red and green channels.
- CoolLED pE-300ultra LED lighting system that can be activated by TTL pulse for experiments requiring optogenetic stimulation.
- 16x and 60x water immersion objectives.
- Electrophysiology system: Multiclamp 700B amplifier, Digidata 1550B analog-digital converter, Clampex data acquisition software, Clampfit analysis software.
Applications
Some of the phenomena that can be identified with this technology include:
- Imaging calcium in dendritic spines at the same time as the electrical activity of the neuron is recorded.
- Visualization of cell morphology and structural synaptic plasticity.
- Visualization and monitoring of intracellular protein movement.
- In vivo experiments in small organisms, such as the nematode Caenorhabditis elegans.
Description
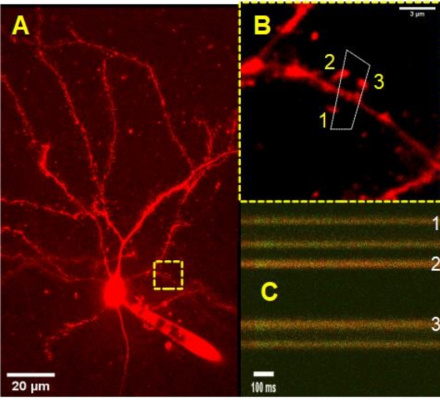
Two-photon excitation microscopy (2PE) enables high-resolution, high-sensitivity fluorescence microscopy in intact neural tissue. In the last 10 years, the applications of 2PE, including microscopy and photostimulation, have contributed to the understanding of a wide range of neurobiological phenomena, since it makes it possible to observe neuronal activity in the living animal and to know the ultrastructural and cellular changes in normal and pathological conditions.
In image A, a pyramidal neuron of the medial prefrontal cortex is observed, which was filled with a red fluorescent dye. The image of this neuron was acquired from a live slice of mouse brain. In B you can see three spines housed in the same dendrite. In C we can see an example of a linear scan of the area marked in B. This image was acquired by Camila Morales using a 60x immersion objective.
MODEL: JEOL JEM1400-Plus
The transmission electron microscope (MET) is a microscopic technique where a beam of accelerated electrons is conducted in a vacuum chamber through a suitably prepared ultra-fine sample, using electromagnetic lenses, interacting with the sample as it passes through it. . Some of the electrons bounce off or are absorbed by the object and others pass through it. All electrons are conducted and modulated by lenses to form a final image on a charge coupled device forming an enlarged image of the sample. The information obtained is an image with different intensities of gray that correspond to the degree of scattering of the incident electrons.
Specifications
- Resolución 0.2 nm (HC) 0.14 nm (HR)
- Tensión de aceleración de 10 a 120 kV
- Aumento 150 a 1.000.000 x
- Camera digital Gatan de 4 MP
Applications
It is used in the observation of biological specimens that include macro-molecular materials, drugs, pathological sections and viruses. Typically, the full view of tissues, structures, target locations, and viewing area are first confirmed at low magnification, and then fine structures of interest are carefully studied at high magnification.
Description
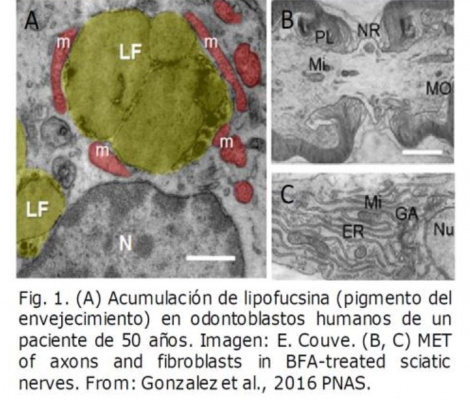
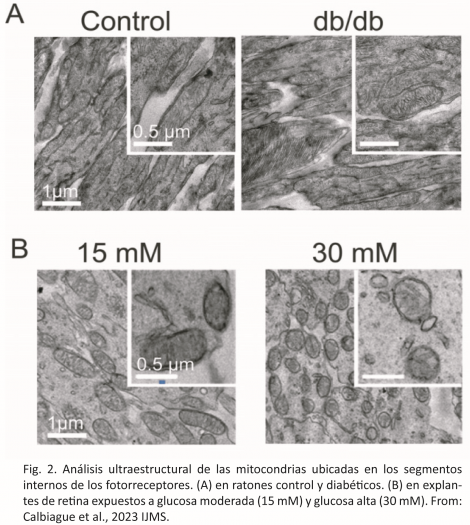
Transmission electron microscopy is a fundamental study tool in biology, since it allows the visualization and analysis of tissue and cellular ultrastructure, at levels of nanometric resolution. The high resolution capacity of transmission electron microscopy is essential for the advancement of biomedical sciences, because physiological or pathological changes in organisms are always based on alterations at the sub-cellular and molecular level.
Immunolabeling techniques with gold particles, with or without amplification by silver, allow the identification and localization of cellular components at unsuspected levels, and have no limits of application. As an example, the detection of the neurotransmitters GABA and glutamate was achieved in sea anemones using this methodology (Delgado et al., J Morphology. (2010)
Transmission electron microscopy is also essential in the areas of diagnosis, pathology, virology and studies of bacterial colonies. For example, using transmission electron microscopy we have identified and characterized the autophagic-lysosomal system in human odontoblasts, establishing correlates that allow us to understand organelle turnover and cell survival strategies through autophagy and isolation of cellular debris (Fig. 1A).
In a recent investigation, members of the laboratory of Dr. Oliver Schmachtenberg have analyzed the ultrastructure of the internal segments of the photoreceptors in search of morphological alterations of the mitochondria (Fig. 2 A and B).
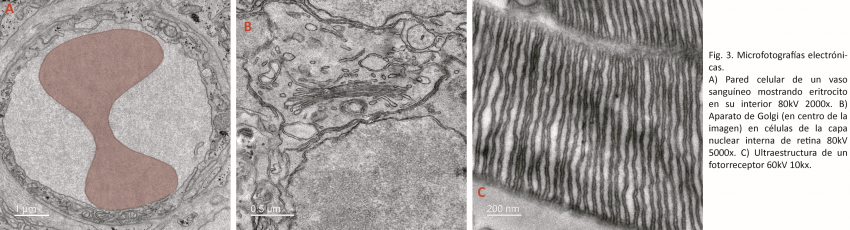
This type of microscopy also makes it possible to study tissues (Fig. 3A), intracellular organelles (Fig. 3B) or specialized cells (Fig. 3C), among other applications in material sciences.
Microscopy LASER-TIRFM (Total Internal Reflection Fluorescence Microscope)
Total internal reflection fluorescence microscopy (TIRFM) is a special technique of fluorescence microscopy that allows to selectively excite fluorophores such as green fluorescent protein (GFP), membrane stains, antibody-bound fluorochromes, among others; in restricted regions of a sample that are immediately adjacent to the interface between the slide (glass) and the solution containing it. This occurs when the incident light is reflected internally in its entirety at the interface of glass and water, generating the so-called evanescent waves, which form an electromagnetic field that affects the fluorophores and decreases exponentially from the interface, and therefore penetrates a depth up to 100 nm from the glass surface. This allows a selective visualization of cell surface regions such as the membrane and events that occur on it, in living cells, being observed with a high axial resolution, proving to be an important tool in biophysics and quantitative biology.
Specifications
- Inverted motorized microscope (Eclipse Ti-E, Nikon)
- Objective (CFI Apochromat TIRF 60X / 1.49 NA and 100X / 1.49 NA, Nikon)
- LASER (488-20LS, OBIS, Coherent)
- LASER (LASER DPSS unit (Coherent compass, 561nm 20mW))
- C11440 Camera (ORCA-FLASH 2.0; Hamamatsu Photonics)
- Software (NIS-Element viewer 4.3, Nikon)
Applications
TIRF microscopy is an excellent technique for combining kinetic studies with spatial information in live or in vitro samples. It is applied routinely to investigate phenomena associated with membranes such as:
- Cell adhesion on different surfaces
- Hormone binding
- Transport of membrane proteins (eg channels or receptors) to the plasma membrane.
- Processes of exocytosis or endocytosis (for example: release and capture of neurotransmitters)
Description
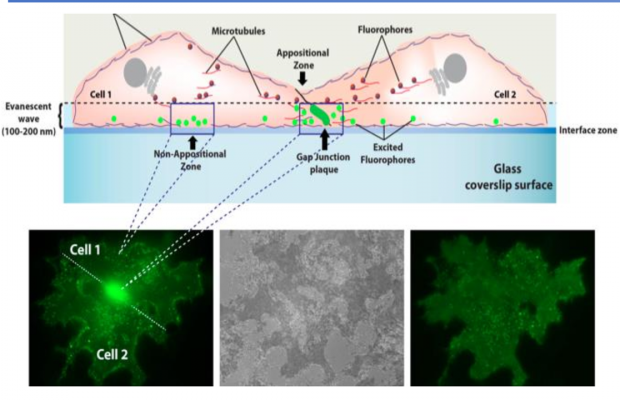
Example of use in CINV: By using spatially restricted evanescent field to excite fluorophores, TIRF microscopy allows observation of the location and dynamics of molecules and processes in an optical section very close to or within the plasma membrane. In the Laboratory of Dr. Agustín Martínez this technique has been used to study the traffic of wild and mutant connexins towards the cell membrane (ex: Cx43EGFP, Cx26GFP).
Connexins are a family of proteins that form cleft junction channels (UH). These connect the cytoplasm of adjacent cells by coupling, head-to-head, of two hemichannels (HC) that are hexameric arrangements of connexin monomers.
Mutations in connexin 26 (Cx26) have been associated with hereditary deafness. It has been suggested that these mutations may alter the properties of the UH channels they form, including permeability, selectivity, and opening and closing mechanisms.
The figure shows the preferential directionality of the Cx43 HCs traffic towards Hot-spots in the cell apposition membrane regulated by actin cytoskeleton. The experimental model consists of TIRF analysis combined with a special type of phase contrast microscopy (SIRC; surface interference reflection constrast) to determine the position of the plasma membrane (dark areas).
L-RET (Lanthanide-based resonance energy transfer) is a spectroscopic technique, a variant of the Förster resonance energy transfer (FRET) technique, very sensitive, based on the transfer of energy from a donor to an acceptor, which depends on the distance that separates these spectroscopic probes, so this technique is very useful for studying conformational changes in proteins within the distance range of 10-100 Å. L-RET uses luminescent lanthanides, most often terbium chelated (Tb3 +) as a donor rather than conventional fluorophores, offering advantages such as a longer shelf life of donor-only emissions, the flexibility to use multiple acceptor fluorophores, and the opportunity to detect the emission of sensitized acceptors in a simple way to measure energy transfer without the risk of also detecting the donor signal.
Specifications
- Quanta-Ray Lab-Series Laser (Spectra Physics)
- The laser excitation beam passes through an excitation optics composed of a fused silica Pellin-Broca prism (Thorlabs) and UG11 cleaning filter, followed by a custom 266nm dichroic laser without anti-reflective coating (AR) (Z266rdc; Chroma Technology)
- Immersion objective 40x (1.25 N.A.) without AR coating (Partec GmbH)
- E515LPv2 long pass or D520 / 20m band pass emission filters (Chroma Technology) for DO broadcasts or SE recordings, respectively.
- Gated photomultiplier tube (Hamamatsu)
- Eight pole Bessel filter
Applications
This technique has been used successfully to:
- Measure intramolecular distances such as amino acids between proteins, which allows determining their spatial shape.
- Track structural changes in proteins such as voltage-gated K + and Na + channels by evaluating their functionality.
- Studies of protein-protein interactions in cells
- Assays to measure peptide dimerization associated with transcription factors and ligand-receptor interactions.
- Más información sobre este texto de origen
Description
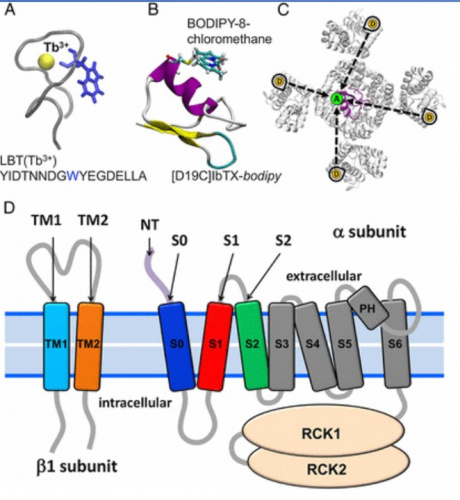
Using LRET, it has been sought, for example, to measure intramolecular distances in the K + channel activated by Ca2 + (BK), one of the most widely expressed channels in mammals; in the absence and presence of integral membrane proteins, called β subunits, and determine the conformational changes induced by depolarization.
In Dr Ramón Latorre’s laboratory, they used a genetically encoded lanthanide-binding tag (LBT) to bind terbium as a LRET donor and a fluorophore-labeled iberiotoxin as a LRET acceptor for distance measurements within the BK channel structure in a living cell. with the strategy presented in the figure. (Carrasquel-Ursulaez et al., Biophysical Journal (2018)).
Contact:







